The Living Lab Bench: The Role of 3D Bioprinting in Smarter Drug Development
What if we could print living tissue? Not just a cast or prosthetic, but living tissue—a patch of functioning heart muscle, a sliver of brain microvasculature, or even an entire organ tailored to your body. This isn’t a sci-fi pitch—it’s the real, rapidly advancing world of 3D bioprinting, and it’s set to fundamentally change how we discover drugs, treat disease, and even perceive the limits of our own biology.
At its core, bioprinting is a bold fusion of two fields: the mechanical precision of 3D printing and the biological complexity of living cells. Instead of plastics or metals, bioprinters use "bioinks"—blends of living cells, hydrogels, and signaling molecules—to recreate tissue-like structures, layer by layer. Although printing entire organs is still out of reach, bioprinted tissues like blood vessels, skin, cartilage, and miniature hearts can already mimic disease, predict drug response, and one day may repair or replace damaged organs.
The Cost of Cure: Why We Need a Printed Path Forward
Drug development is notoriously inefficient. It takes 12 to 15 years to bring a new drug to market, costs up to $2 billion, and has a staggering 90% failure rate in clinical trials. In cancer research alone, an estimated $60 billion is lost every year on drugs that don’t make it to patients.
Additionally, More than 115 million animals are used in drug testing each year, even though animal models are often poor predictors of how drugs will work in humans. We've relied on them because they were the best tools available. But what if we had something better?
Researchers at the forefront of bioprinting now envision a new paradigm for drug development powered by lab-grown tissues. Instead of testing compounds in animals or generic cell cultures, scientists can bioprint miniature human tissues—complete with blood vessels and disease-specific architecture—to simulate how drugs behave in the body.
High-resolution bioprinting, which uses lasers to sculpt ultra-fine tissue structures, allows us to model disease at a microscopic level and study drug effects in detailed tissue environments. In contrast, low-resolution bioprinting sacrifices fine detail for size—constructing larger tissue sections (on the centimeter scale) that serve as implantable scaffolds in regenerative medicine.
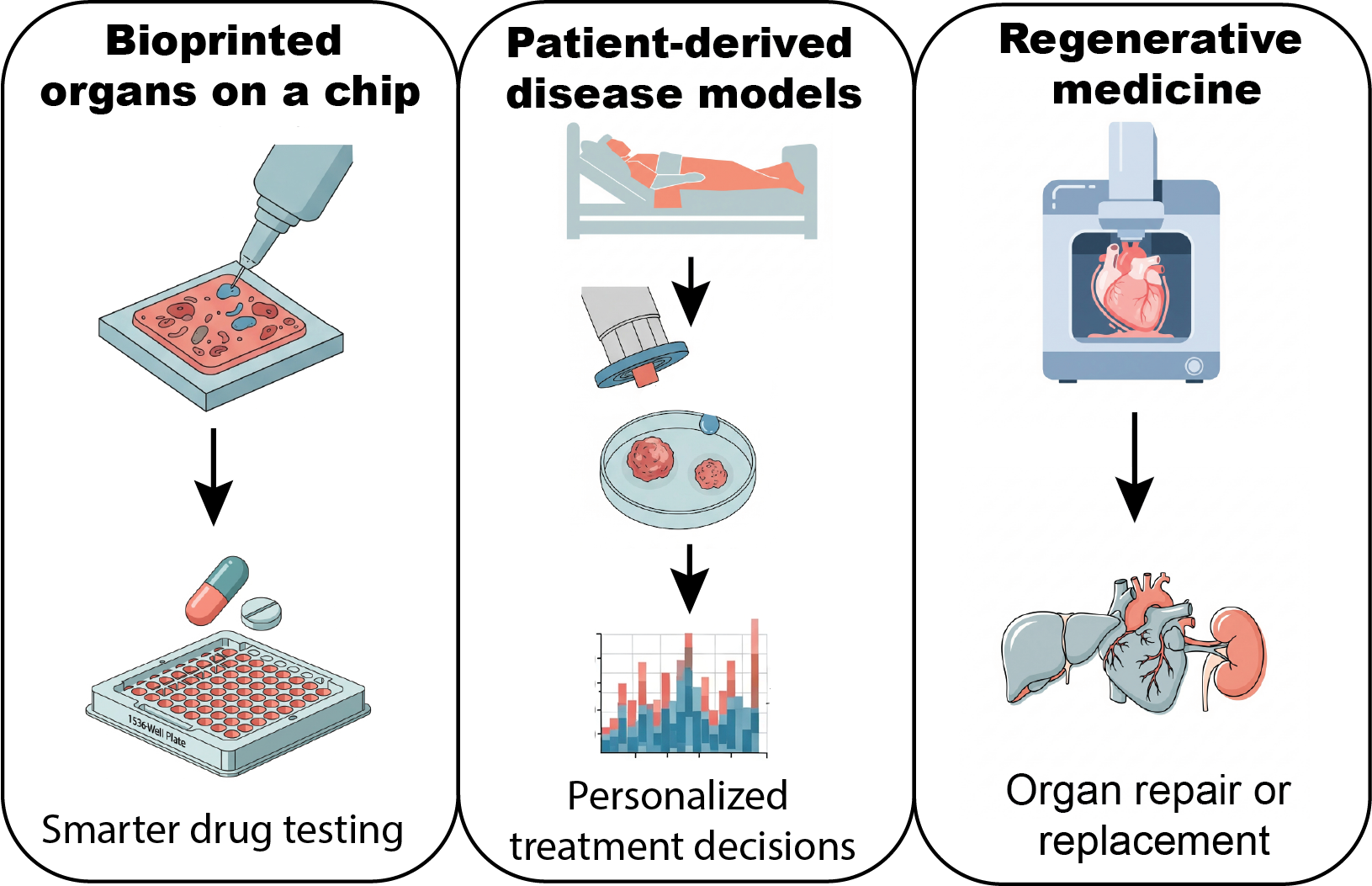
Together, these technologies enable three transformative applications: smarter drug development, personalized treatment, and regenerative medicine.
This illustration highlights the core applications of 3D bioprinting in biomedical research. By enabling the creation of human-relevant tissue models, 3D bioprinting is accelerating drug discovery and improving the accuracy of preclinical testing. It also opens new avenues for personalized medicine using patient-derived tissues and supports regenerative medicine through functional implants, such as cardiac patches.
1. Smarter Drug Development
By creating vascularized, disease-mimicking tissues in the lab, researchers can simulate how drugs are absorbed, distributed, and metabolized in the human body—without involving a single animal. This allows scientists to: flag ineffective or toxic drugs before they reach clinical trials, test multiple drug candidates in parallel, and reduce dependency on animal testing (a shift supported by policy, such as the FDA Modernization Act 2.0, which eliminates the requirement for animal testing before human trials).
By printing into prefabricated scaffolds or chips, researchers can also spatially arrange different cell types to create heterogeneous tissue structures: vascular networks, liver lobules, neural circuits. Think of it as printing functional blueprints of organs—just on a smaller, chip-sized scale. This has given rise to complex organoids (mini-organs grown from stem cells) and multi-organ systems, often referred to as multi-organs-on-a-chip. These lab-grown models mimic the structure, function, and even disease pathology of human organs, enabling researchers to: study disease progression in real time, track how a drug moves from one organ to another, and test cancer therapies on tumoroid arrays—3D models that closely replicate real tumors.
With this level of fidelity and miniaturization, we’re moving toward integrated platforms that can host multiple printed organs in a single system—with improved biosensing, reduced footprint, and the potential to simulate entire physiological pathways.
In short: we’re not just bioprinting tissues anymore—we’re bioprinting systems. Tiny, complex, human-relevant systems that are redefining how we develop and test treatments.
2. Personalized Medicine: Printed Just for You
No two patients are alike—and neither are their diseases. Take breast cancer: two patients with the same diagnosis may respond completely differently to the same drug. Bioprinting opens the door to truly personalized medicine.
Imagine taking a small biopsy from a patient, bioprinting a mini-tumor that mimics their exact cancer, and testing all available therapies directly on it. Doctors could select treatments based on real-time data from a patient’s own tissue—not population averages. It’s like designing a clinical trial tailored to one individual.
3. Regenerative Medicine: Healing by Design
Perhaps the boldest promise of bioprinting lies in regeneration. Using low-resolution techniques, scientists can now print tissues that are not only alive—but functional. Cells that regulate disease, produce hormones, or repair damage.
For instance, researchers can now print living cardiac tissue patches to treat patients with heart failure. These patches, derived from stem cells, beat rhythmically like real heart muscle and can integrate into damaged areas to restore lost function—potentially avoiding the need for full heart transplants. Similar efforts are underway to repair injured livers and lungs.
The Dream: Printing Whole Organs
Every year, hundreds of thousands of people wait for organ transplants—kidneys, hearts, livers—each hoping for a call that may never come.The demand for donor organs is relentless, but the supply is limited. What if we stopped waiting? What if, instead of finding a match, we could print one?
Can we print an entire organ in the lab—custom-designed, rejection-proof, and ready for implantation? Technically, not yet. No current platform can fully replicate the intricate architecture, vascularization, and cellular diversity of a real organ.
The challenge is complex. Too much pressure during printing and cells are crushed. Too little, and the structure collapses. Time is critical—you must print quickly enough to keep cells alive, but carefully enough to preserve function. And printing is just the beginning. Cells must also communicate, adapt, and function inside a living body.
The roadmap is clear: start with simple tissues, build toward increasing complexity, and eventually integrate everything into a full organ. It’s not a question of if, but when.
As with any new frontier, there are ethical, regulatory, and technical challenges ahead. But for those of us at the edge of biotech, bioprinting represents something exhilarating: a future where biology is no longer just observed—it is designed, layer by layer.
Would you like to stay updated on the latest breakthroughs in biomedical science? Subscribe to my blog and join me in exploring the next frontier of medicine!