Undruggable No More
Drugs are usually chemical molecules that bind to a protein target to modulate its function and as a result treat symptoms or cure disease. For decades, drug discovery has largely focused on the “low-hanging fruit” of the human proteome—proteins with deep pockets and well-defined structures that small molecules can easily latch onto. Think kinases, GPCRs, ion channels—targets tailor-made for medicinal chemistry. But this is only part of the picture. In fact, it’s estimated that only about 40% of the human proteome is currently considered “druggable”, meaning we have the chemical tools and structural footholds to develop effective therapeutics. The remaining 60% falls into the ominous category of “undruggable”: proteins with flat surfaces, disordered regions, extreme structural similarity to other proteins, or essential functions that make them difficult to target without harming healthy cells. Historically, this label meant “not worth pursuing.” Today, it means “challenge accepted.”
How We Develop Molecules to Target Proteins
At its core, drug discovery is about finding a molecule—a small compound, a peptide, or a biologic—that binds to a protein in just the right place and changes its function in a way that benefits patients. The process is iterative. Identify a target linked to disease. Find a binding site where a molecule can interact. Design or screen for compounds that bind. And optimize for potency, selectivity, and safety.
For “druggable” targets, this workflow is straightforward because there’s usually a clear binding site. For the “undruggable,” step two is often the stumbling block—no pocket means no obvious way in.
Why Proteins Are Considered Undruggable
Some proteins earn the “undruggable” label for a mix of tricky reasons. Sometimes there’s just no obvious place for a drug to latch onto—imagine a flat, featureless surface where a small molecule can’t find any grip. Others look too much like their relatives, so targeting them risks hitting the wrong protein and causing side effects. Some are so vital to normal cell function that shutting them down could do more harm than good. Then there are the shapeshifters—intrinsically disordered proteins that never settle into one structure—and those that only appear briefly in fleeting partnerships, making them tough to catch in action. Well-known members of this elusive club include KRAS, Myc, certain transcription factors, protein–protein interaction hubs, and phosphatases.
Breaking the Rules: New Strategies to Tackle the Undruggable
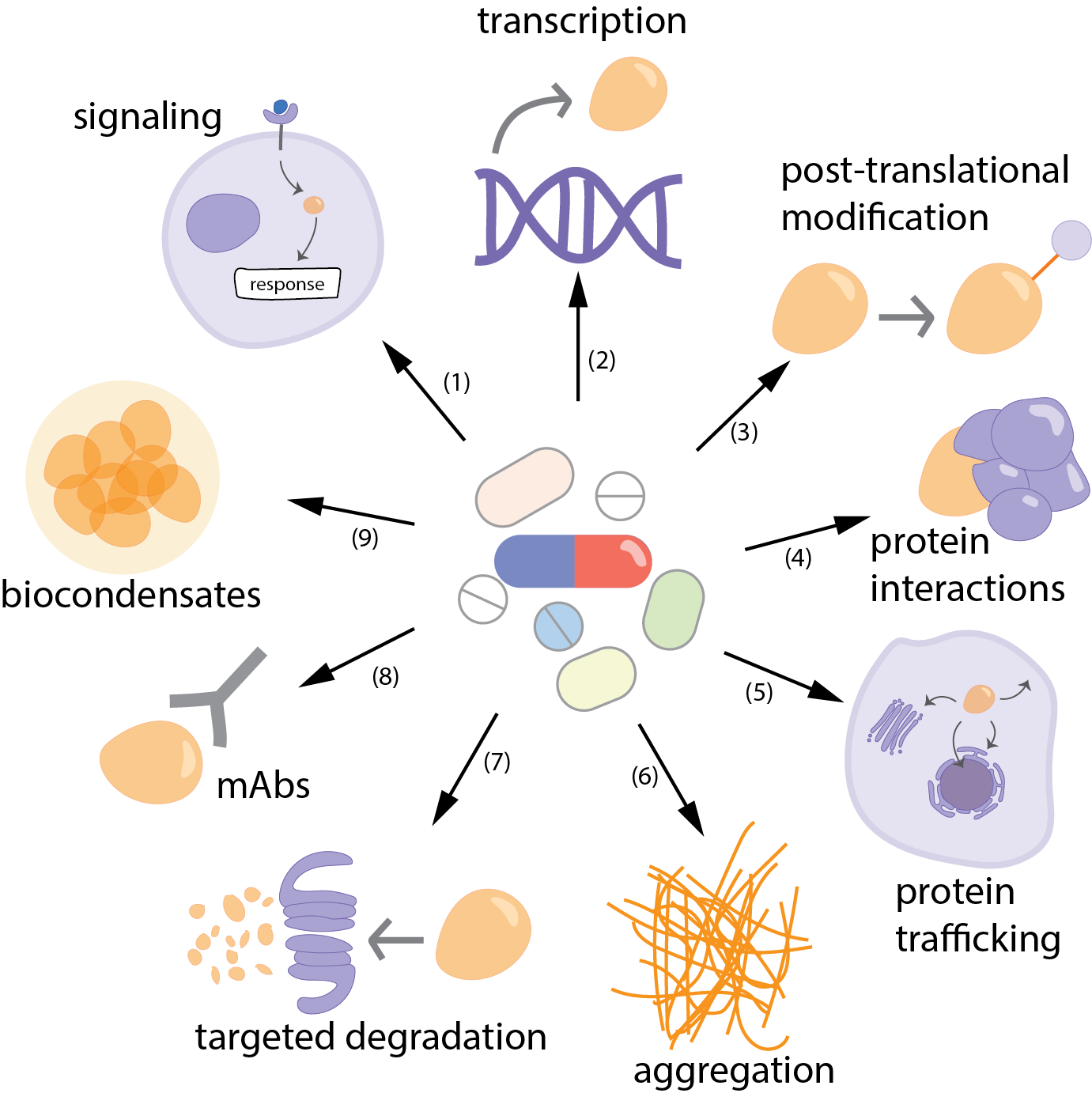
Over the past decade, researchers have pushed beyond the limits of traditional drug design, moving away from the idea that a protein must present a perfect “lock-and-key” pocket to be considered druggable. Instead, they now interfere with protein function and disease pathways at multiple levels: controlling upstream signals that trigger overexpression, blocking transcription, modulating post-translational modifications, disrupting harmful interactions, redirecting proteins to different cellular locations, or preventing toxic aggregation in diseases such as Alzheimer’s.
Besides, when direct targeting is necessary but challenging—as with proteins that lack stable structures or accessible binding pockets—innovative solutions have emerged. PROTACs, for instance, bypass the need for deep binding grooves by tagging proteins for degradation, while antibodies and other biologics reach broad or shallow surfaces beyond the reach of small molecules. Allosteric modulators fine-tune protein activity from alternative angles, and covalent inhibitors lock onto unique amino acids with permanent bonds, as seen with KRAS-G12C inhibitors like sotorasib. Even intrinsically disordered proteins, once dismissed as undruggable, can be tackled indirectly by targeting higher-order assemblies such as biomolecular condensates.
New technologies are widening the scope even further. Artificial intelligence now predicts hidden binding sites, simulates protein dynamics, and explores chemical space with unprecedented reach. Fragment-based and DNA-encoded libraries identify weak starting points that can be optimized into potent inhibitors, while nucleic acid therapeutics—from antisense oligonucleotides to siRNA and mRNA-based drugs—bypass proteins entirely by acting at the genetic level.
These strategies are already transforming drug discovery. KRAS-G12C, once thought impossible to inhibit, can now be silenced with covalent inhibitors that exploit a concealed pocket near the mutant cysteine. Mutant p53 can be stabilized to regain its tumor-suppressor role. The BCL-2 family, long considered untouchable protein–protein interaction hubs, is now targetable with drugs like venetoclax in leukemia. Even BET bromodomains, lacking obvious pockets, can be blocked or degraded.
The common thread is a shift in mindset: instead of forcing proteins to fit an old model, scientists are redesigning the locks, inventing new keys, and even recruiting the cell’s own systems—like proteasomes and the immune machinery—to do the work. This shift has expanded what it means for a protein to be “druggable” and opened once-closed doors to entirely new therapeutic possibilities.
Strategies to tackle “undruggable” proteins. A variety of approaches can be used to interfere with disease processes driven by proteins that are difficult—or so far impossible—to drug. These include: (1) targeting upstream signaling pathways, (2) blocking transcription, (3) modulating modifying enzymes, (4) disrupting protein–protein interactions, (5) altering localization, (6) preventing aggregation, (7) targeted degradation (8) monoclonal antibodies (9) targeting condensates for disordered proteins.
Conclusion and Perspective
The phrase “drugging the undruggable” is quickly becoming outdated. Advances in structural biology, chemical biology, and computational modeling have blurred the boundaries between what can and cannot be targeted. Today, undruggable really means “not yet drugged.”
The next decade will likely see an expansion of therapeutic modalities—from designer degraders to programmable RNA drugs—turning more “no-go” zones into active drug development pipelines. For patients, this means hope for diseases that were once considered beyond the reach of modern medicine.
Would you like to stay updated on the latest breakthroughs in biomedical science? Subscribe to my blog and join me in exploring the next frontier of medicine!